HeV sampling equipment and procedures
Veterinarians should only undertake sampling to obtain a Hendra virus (HeV) diagnosis, if safe to do so.
Make sure you are familiar with the below procedures for:
- entering and exiting a contaminated site
- taking samples with the required equipment.
Check the clinical signs and types of tests listed in the veterinary information for HeV.
Sampling requirements and equipment
Different samples are needed from live and dead horses to test for HeV.
Taking samples from multiple sites for each horse will increase the diagnostic sensitivity of the procedure. Consider taking duplicate samples for further diagnostic work, if the tests are HeV negative.
Live horses
Live horses
Preferred samples
- Nasal, oral (tongue surface), and rectal mucosal (not faecal) swabs.
- Blood – 1x 10ml serum tube, 1x10ml EDTA tube.
- Urine swab, taken from the ground immediately post-urination if possible.
Sampling equipment for each live horse
- shielded vacutainer needle and holder, plus several spares
- 1x serum tube, 1x EDTA tube, plus spares
- 3 x virus transport media (VTM), plus spares – if VTM is not available, place swabs in small amount of saline
- 4 x swabs, plus spares
- sharps disposal container.
Dead horses
Dead horses
Preferred samples
- Nasal, oral, rectal mucosal swabs.
- Blood, if available – 1x serum tube, 1x EDTA tube.
- Additional necropsy samples:
- blood clot taken from cutting the jugular vein
- submandibular lymph node tissue.
Sampling equipment for each dead horse
- shielded vacutainer needle and holder, plus several spares
- 1x serum tube, 1x EDTA tube, plus spares
- 3 x VTM, plus spares
- 4 x swabs, plus spares
- scalpel, scissors, and forceps to collect tissue samples
- sample jars for fresh tissue samples in formalin
- cut-resistant gloves (Kevlar) may be considered.
Make sure you have skilled assistants to handle the horses and seek further help with sampling if necessary.
Do not place yourself or assistants at risk of injury at any time. Use the following safety equipment and disinfectants.
Personal protective equipment (PPE)
Personal protective equipment (PPE)
PPE for each person
- disposable overalls with at least splash resistant rating, plus a disposable or washable hat if the overalls have no hood
- one of the following respirator options:
- disposable P2 or N95 particulate respirator, plus spares
- reusable negative pressure respirators
- powered air purifying respirator (PAPR) and filters
- pack of disposable gloves – nitrile gloves recommended
- safety eyewear or a face shield
- roll of duct tape
- pair of impervious rubber boots.
Respiratory requirements
We recommend discussing specific respiratory protection needs with a supplier of safety equipment.
The minimal level of respiratory protection when investigating a potential HeV case is P2 particulate respiratory protection.
Disposable particulate respirators must be fitted correctly and are not suitable for people with facial hair. These people should use a powered air purifying respirator (PAPR) which draws air through a filter and supplies it to a hood worn over the head.
Overalls
Splash-proof overalls are lighter and suited to hotter conditions that may be faced during investigation.
Impervious overalls present a risk of overheating in less than 20 minutes, particularly if used in direct sunlight.
Decontamination equipment
Decontamination equipment
Waste disposal
- small sharps container with built-in needle removal facility
- 2x A4 clip seal bags to remove samples from premises
- plastic bucket for carrying equipment
- 1x 500 ml spray pack of Virkon, or other approved disinfectant.
Disinfection
- foot bath and 2 or 3 buckets
- scrubbing brush
- hoof pick or medium screwdriver
- 20 litres water
- disinfectant sachets or bulk supply for mixing at 50g per 5L water, such as Virkon or Trigene
- soap or detergent
- small hand sprayer for chosen disinfectant – if using reusable respirators
- 1x heavy duty garbage bag, plus 1x clinical waste bag
- 2x zip or cable ties
- ground sheet or plastic mat, no more than 1 square metre.
Approved disinfectants
Approved disinfectants
HeV is a member of Category A viruses, which contain a lipid envelope.
The following disinfectants are recommended for either their effect against all viruses, or their specific action against Category A viruses. Use these when handling horses that may have HeV:
- soaps and detergents
- Virkon
- hypochlorites
- iodophors, iodine
- biguanidines such as chlorhexidine
- quaternary ammonium compounds.
Other disinfectants are considered effective, but they require special precautions for safe use. See the AUSVETPLAN decontamination manual for further information.
Sampling procedures
At the entry and exit point of the property, identify the following:
- a clean area
- a dirty area – the contaminated area where the suspect horse is
- a small transition area.
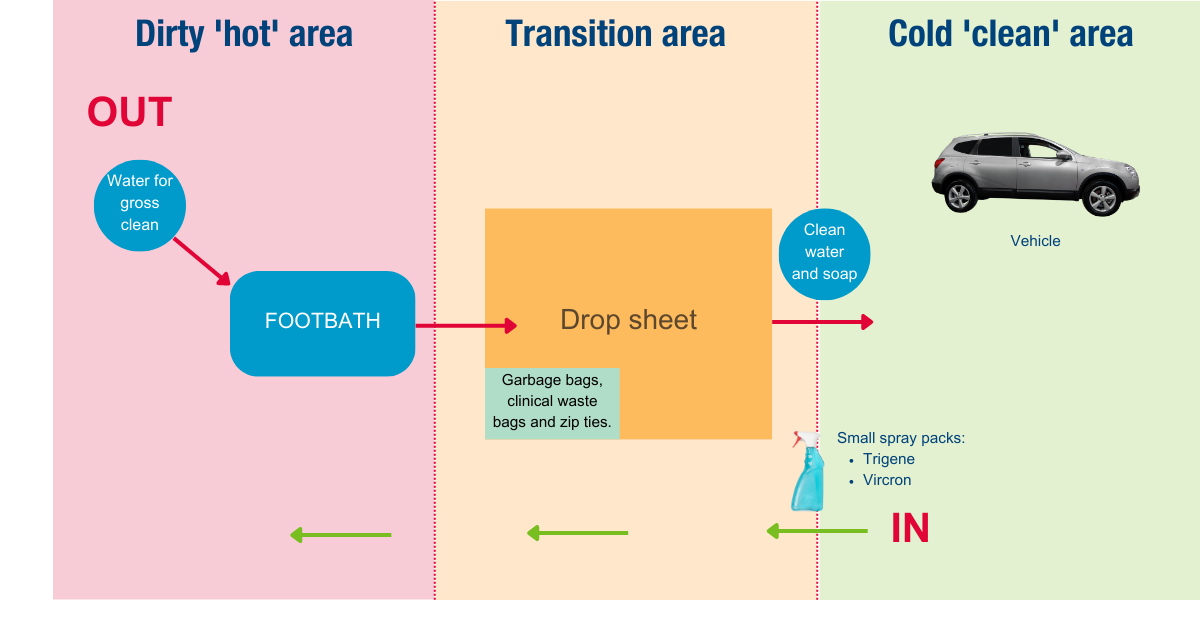
Setup an entry and exit site
On the clean side, lay out and double check you have all the equipment required for the investigation.
Gather the gear needed for sampling and make sure nothing unnecessary will be taken into the dirty area.
Place the disinfection equipment, including containers of disinfectant, soap and clean water, in the transition area for later use.
Put on PPE
Put on PPE
Enter the dirty area only after you are fully dressed in PPE and have all the required gear ready. Follow this sequence:
- Wash hands with soap or detergent.
- Put on overalls then boots – overall legs go outside the boots.
- Put on safety eyewear, gloves and disposable respirator – if using a PAPR instead, put this on, along with gloves, after step 5.
- Fit check the respirator.
- Pull overalls hood up if present and zip to chin – if the overall have no hood, put on a protective hat.
- Double-glove and tape the outer gloves onto the sleeves of the overalls.
Anyone assisting you, or nearby, must wear the same standard of PPE.
Collect samples
Collect samples
It is critical to minimise the chance of contaminating people and their PPE. Follow these precautions when taking samples:
- Clearly and uniquely label all specimens.
- Undertake safe sharps handling and waste disposal – use the sharps container and do not re-cap needles.
If you're accidentally exposed to blood or body fluid, or sharps injury: Wash the affected area of skin thoroughly with soap and water, or irrigate mucous membranes with water or saline. Seek immediate medical advice.
- Once sampling is complete, place in a clip seal bag for removal.
Avoid spreading contamination
Follow these steps before entering the designated clean area:
- Remove gross contamination from yourself and equipment before reaching the entry and exit point. Use a brush and soap, or detergent and water.
- Clean the treads of the boots with a tap on site, or the bucket in the transition area.
- Go to the dirty side of the transition area to double-bag the samples in clip seal bags.
- Disinfect the samples on the clean side – be careful not to contaminate them with disinfectant.
- Spray disinfectant on the outer gloves.
Remove and disinfect PPE
Remove and disinfect PPE
Depending on the type of respirator used, there are different procedures for removal, as below:
- Remove the outer pair of gloves to garbage bag.
- Wash hands, still encased in the inner pair of gloves, in disinfectant.
- Remove boots and stand on drop sheet in the transition area.
- If using a P2 respirator or a negative pressure full-face respirator, then:
- Remove overalls to garbage.
- If wearing a hat, either remove it to garbage bag, or soak in disinfectant, seal in a bag and remove for laundering.
- Remove and disinfect safety eyewear.
- Remove respirator and either throw disposables in garbage, or wipe reusable respirators with disinfectant solution.
- Remove inner gloves to garbage.
- If using a PAPR, then:
- Remove and disinfect the PAPR.
- Remove overalls to garbage.
- Remove the inner gloves to garbage.
Decontaminate equipment
Take care not to re-enter the dirty area while disinfecting equipment and follow these steps:
- Place all waste material in garbage bag, seal it with zip tie, and disinfect surface.
- Double-bag waste by placing garbage bag into a clinical waste bag, seal with zip tie, and disinfect surface.
- Disinfect and double-bag non-disposable items for removal.
- Put on street shoes.
- Pack up disinfection site, disinfecting all equipment thoroughly as packed.
- Wash disinfectant off reusable respirators with clean water.
- Wash hands and other exposed skin with soap and water.
If non-disposable PPE cannot be adequately decontaminated on site, double-bag it and remove for later attention – this is not a preferred option.
Learn how to safely package and send samples for HeV testing.
Precautions after sampling
We recommend minimising interaction with other animals or people until you have done the following:
- Wash exposed areas of skin thoroughly with soap and water.
- Remove and wash dirty clothes in separate hot wash cycle with detergent – do not wash potentially contaminated clothing with other household laundry.
- Take a hot shower with shampoo and soap.
- Dress in clean clothes.
- Put on clean footwear, or footwear not worn in the dirty area on the site.
Provide biosecurity information
Provide biosecurity information
Before leaving the property, discuss these necessary biosecurity matters:
- Enquire as to the amount of human contact people on the property have had with the sick or dead horse.
- Advise the property owners or managers on:
- appropriate biosecurity processes and PPE
- isolating sick or dead horses from people and other animals, including pets
- limiting movement of horses, horse products, and visiting practitioners on and off the property
- disposing of the horse body on the property – this should only be undertaken by people who are aware of the risks and appropriate methods
- seeking medical advice if unwell or concerned about possible exposure
- contacting SA Health's Communicable Disease Control Branch if needed.
- Inform the Biosecurity division of the suspect Hendra case, or provide an update of situation if already notified.
Isolate potential sources of transmission
Isolate potential sources of transmission
If possible, avoid any risks of spreading HeV until the results are known. This includes:
- contact with other animals and people after leaving the property
- using contaminated equipment that was taken into the dirty area, which should have been decontaminated and double-bagged in the transition area.
If the equipment cannot be isolated until the HeV test results are returned, wear PPE to clean it, using detergent then disinfectant. Be careful when removing the PPE.
If the results are negative, clean equipment per the standard practice routine. If results are positive, contact PIRSA's Biosecurity division.
Contacts
- PIRSA, Biosecurity division, Animal Health
- Gribbles Vetlab: 8202 3300 (24 hours)
- SA Health: 1300 232 272 (24 hours, 7 days)
- Health Direct: 1800 022 222
- SafeWork SA Help Centre: 1300 365 255
Information on this page has been sourced with permission from the Queensland Government Hendra virus information for veterinarians.
